Recent LegitScript data reveals the skyrocketing promotion of both GLP-1 medications and reported ads associated with selling compounded GLP-1 medications (press release).
LegitScript tackles the sale of problematic healthcare products as well as more than 60 other high-risk categories — including counterfeits, weapons, and online gambling — with a multi-pronged approach that employs big data, AI-powered technology, and human expertise. This includes helping major platforms and e-commerce marketplaces remove misleading advertisements and bad actors operating in a variety of industries. LegitScript also protects payment facilitators, ISOs, and acquirers from violative merchants of all types, and works with merchants operating in highly regulated industries to help them ensure compliance and build trust. By working with all key players, LegitScript helps to foster a safer online environment for consumers.
The following FAQ is intended to help consumers and the media better understand the issues and risks around the purchase of GLP-1 medications online. For more information, contact us.
What is currently happening in the GLP-1 / weight loss drug market?
After certain GLP-1 receptor agonist drugs such as Ozempic and Wegovy were placed on the FDA drug shortage list, specialized pharmacies began compounding semaglutide and other similar peptide-based drugs for patients seeking to use them for weight loss purposes. The shortage of available drug manufacturers and the ability to compound, coupled with current high consumer demand, has led to large numbers of compounding pharmacies supplying customer demand for weight-loss drugs.
However, these compounded medications are not FDA-approved, meaning they haven’t undergone the same rigorous testing process to ensure safety and effectiveness as commercially available options. This raises concerns about their composition, potential side effects, and overall quality. The FDA has repeatedly emphasized the necessity of patients only obtaining weight-loss drugs with a prescription from a licensed healthcare provider, and then only from state-licensed pharmacies or outsourcing facilities registered with FDA.
In October 2024, the FDA announced that the shortage of tirzepatide, the active ingredient in Mounjaro and Zepbound, was resolved, and shortages of other GLP-1s are also poised to be resolved in the near future. That means millions of patients will soon potentially lose access to cheaper, but less regulated, alternatives to the brand name drugs. With compounding pharmacies now unable to broadly manufacture tirzepatide, demand for low-cost versions of this popular semaglutide may outstrip supply. As a result, LegitScript expects to see problematic behavior including illicit compounding, especially from overseas manufacturers, and counterfeit versions of brand name drugs for sale.
For more information on semaglutide, we recommend reading through the FDA’s full prescribing information document here.
LegitScript’s founding mission is to create and maintain a safer, more transparent internet and payment ecosystem, and in the past, we’ve generally seen that when demand for a product is exceedingly high, abuse and compliance violations also increase.
Can you quantify the rise in merchants offering online weight loss medications?
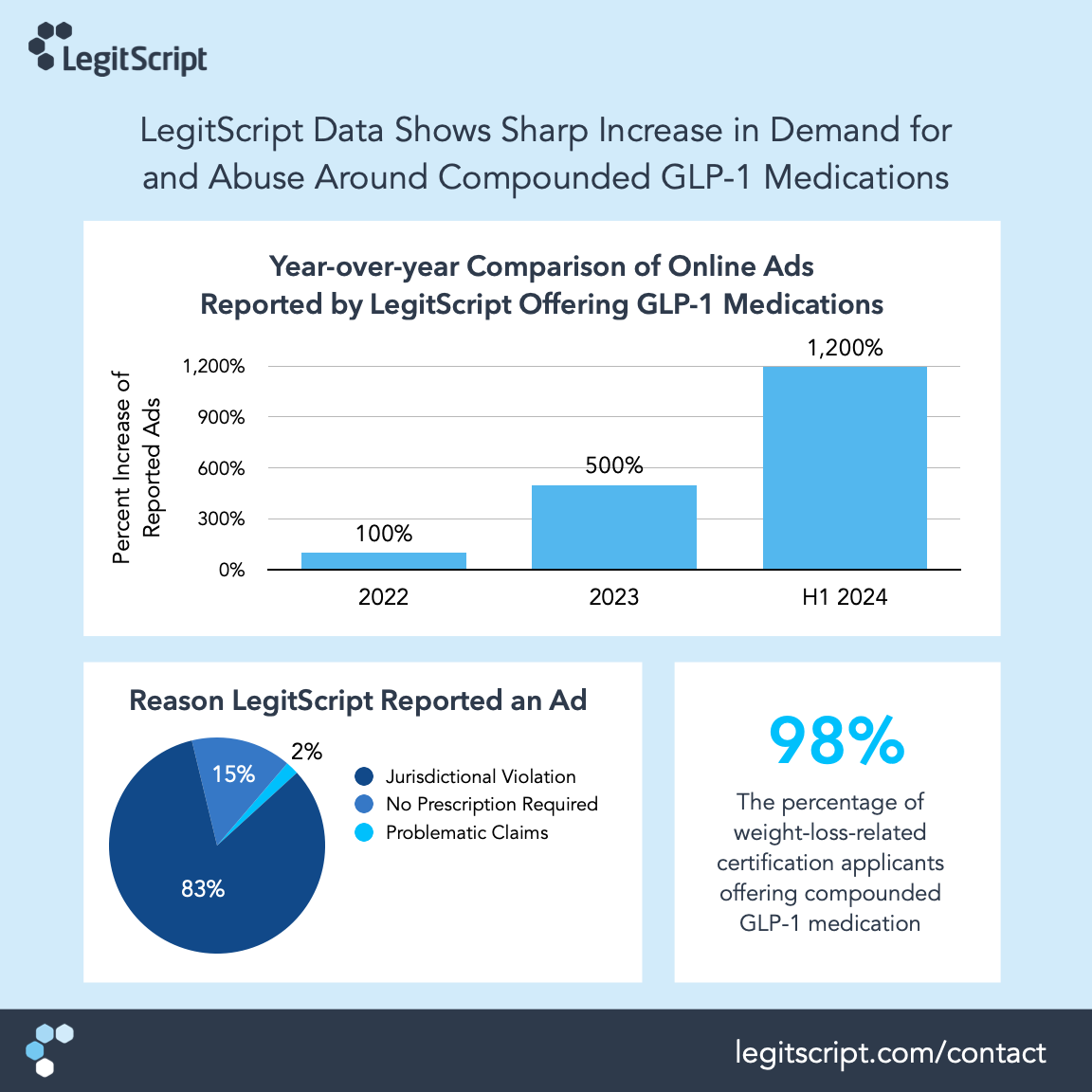
In the first half of 2024, LegitScript has already seen almost as many (94%) new healthcare merchant applications as we saw in all of 2023. For comparison, applications grew 47% from 2022 to 2023. Over 60% of the providers that have applied for certification have a focus on weight-loss medication, and of those, 98% offer compounded GLP-1 medications.
How does the increase in both GLP-1 drug demand and compounded drug options affect the digital marketplace and payments ecosystem?
In order to combat abuse and other violations, LegitScript utilizes a multi-faceted approach:
- Monitoring online merchants, e-commerce marketplaces, and ads for violations
- Reporting violations and high-risk activity to partners
- Certifying new and existing telemedicine providers to ensure compliance
This year, we’ve seen a significant increase in all of our service verticals. In the past two months alone, we’ve monitored more than 500,000 paid search advertisements referencing GLP-1s. Furthermore, we’ve detected a 12x (1200%) increase in violative and/or problematic advertisements since 2022.
This surge in violative activity has raised numerous safety concerns among vendors, regulatory bodies, and patients, which emphasizes the continued need for real-time monitoring as well as thorough certification and compliance from all parties participating in the online digital health ecosystem.
How does LegitScript ensure compliance and legitimacy for new and existing health and wellness providers?
Eighty-three percent of the violative or potentially problematic GLP-1 drug ads we’ve identified and reported were being shown in jurisdictions where advertisements for prescription medicines are not allowed., while 15% of reported ads were offering GLP-1 drug products without a prescription.
This worrying trend not only bypasses essential medical oversight but raises natural concerns about the safety, authenticity, and effectiveness of these medications.
In order to combat these violative ads, LegitScript provides industry-leading certification programs to closely vet and monitor legitimate health and wellness providers of goods and services so they can advertise, process payments, and improve consumer confidence.
Our team of highly skilled researchers and analysts utilizes advanced technology and proprietary data to make and keep the internet and payments ecosystem safer and more transparent.
What content is considered violative?
With the huge increase in GLP-1-related digital merchants and ads, we are constantly monitoring for and reporting violative and problematic practices. We have observed a variety of violations including:
- GLP-1 drugs being advertised in jurisdictions where it is not permitted
- GLP-1 drugs being offered for sale without a prescription
- Scammers offering GLP-1 drugs but not shipping anything
- Compounding pharmacies claiming that their GLP-1 drugs are FDA-approved
Additionally, because compounded versions of these drugs have not been FDA-approved, there is increased risk of problems with safety and efficacy of these products.
How can I ensure my treatments are coming from a certified vendor?
All LegitScript-certified websites have been carefully vetted for demonstrating compliance. The full list of our healthcare certification standards can be viewed here. Additionally, websites are monitored on an ongoing basis for compliance with applicable regulations and LegitScript standards.
Once a business has been certified, their website and product are added to our internal LegitScript database, which members of the public can search to see who has been certified.
To see if a website is LegitScript-certified, all members of the public can access our simple look-up tool. Additionally, once a business is certified, a seal of approval badge is generated and shared directly with the merchant, which they may display on their website.
About LegitScript
LegitScript has been leading the charge for a safer, more transparent internet and payments ecosystem for more than 15 years — combining technology and data with a team of experts skilled in monitoring, certification, and investigations. LegitScript’s broad and deep view across the entire commercial internet provides unique insights from all industries and angles, allowing businesses to evaluate, manage, and mitigate risk. That’s why LegitScript is trusted by the world's largest internet platforms, payments companies, and regulatory agencies.