The FDA has approved drugs like Ozempic, Wegovy, and Rybelus for those suffering from obesity, diabetes, and other weight-related illnesses. Since their approval, these drugs have faced worldwide popularity — resulting in supply shortages. These shortages have caused healthcare merchants to offer alternative products containing semaglutide, the active pharmaceutical ingredient in Ozempic, Wegovy, and Rybelsus. Read more for details about these drugs are why some semaglutide products are considered problematic.
What Is Semaglutide?
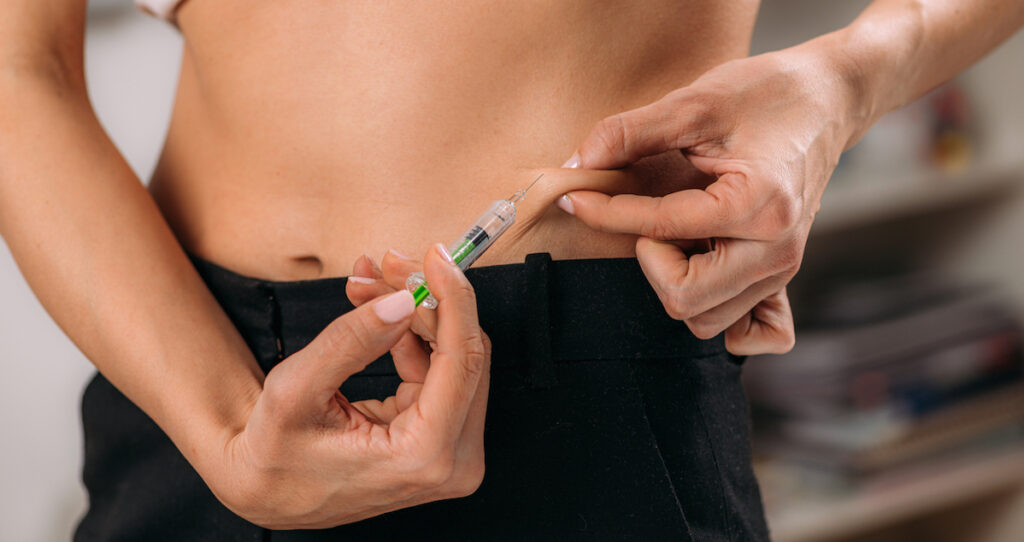
Semaglutide (often sold under brand names such as Wegovy, Ozempic, and Rybelsus) was first introduced in 2017 to manage type 2 diabetes. It works by imitating GLP-1, a naturally occurring hormone, signaling the stomach to empty more slowly. In June 2021, the FDA approved Wegovy (semaglutide) for weight management in overweight or obese adults with co-occurring health issues. Since then, drugs containing semaglutide have risen in popularity across celebrity circles and on social media as an easy way to quickly lose weight.
Semaglutide inhibits glycogenolysis and gluconeogenesis, two processes that increase blood sugar during digestion. As a side effect, semaglutide also decreases hunger and food cravings. Semaglutide medications are taken once daily (or via weekly subcutaneous injections). The most common side effects include but are not limited to digestive issues such as nausea, vomiting, diarrhea, and constipation.
In extreme cases, patients can become dehydrated, need prescription anti-nausea medication, or even need to be hospitalized. It’s essential that a doctor oversees the use of this medication in order to reduce the occurrence of more harmful side effects.
What About Compounded Semaglutide?
Semaglutide has become extremely popular for weight loss, and despite its high price tag (often fetching $890 to $1,300 for a monthly supply), demand has rapidly outpaced supply. Some US patients have even resorted to ordering from international pharmacies to obtain the medication, most often buying from Canada or Mexico (see the image below).
After many brand name GLP-1s were placed on the FDA drug shortage list, specialized pharmacies were granted limited permission to compound semaglutide and other similar peptide-based drugs for patients seeking to use them for weight loss purposes. Many compounding pharmacies, often in partnership with or as part of telemedicine platforms, began creating their own versions of GLP-1s, usually at a much lower price than the brand names. Compounded versions put these drugs within reach of more consumers, which has helped spur even more demand. Nearly all leading telemedicine companies pivoted to offering semaglutide, a type of GLP-1 drug.
The FDA’s allowance for manufacturers to create compounded versions of GLP-1s lasts only as long as there is scarcity, and the administration has begun to remove the affected GLP-1s from its shortage list. In October 2024, the FDA announced that the shortage of tirzepatide, the active ingredient in Mounjaro and Zepbound, was resolved, and shortages of other GLP-1s are also poised to be resolved in the near future. That means millions of patients will soon potentially lose access to cheaper, but less regulated, alternatives to the brand name drugs.

LegitScript Is Seeing Various Kinds of Problematic Behavior With Semaglutide
High demand and reduced availability has created the perfect environment for bad actors to flood the market with unapproved and potentially unsafe products. LegitScript has already observed merchants offering semaglutide to US consumers without requiring a prescription, which poses an elevated risk for regulatory and card network scrutiny.
Now that the FDA’s compounding allowance for GLP-1s is coming to an end, LegitScript expects to see other problematic behavior including illicit compounding, especially from overseas manufacturers, and counterfeit versions of brand name drugs for sale.
We also expect to see more nondelivery schemes (in which fraudsters pretend to sell a product at a discounted price but never actually ship anything). Already, Australia’s Therapeutic Good Administration (TGA) warned citizens about online scams related to semaglutide. Australian officials were seeing advertisements that marketed semaglutide-containing drugs for prescription online to be shipped directly to consumers’ doorsteps.
The TGA stated, “In some cases, consumers who pay for products online fail to receive any product, while in other cases the product received is not semaglutide. Even for those individuals who receive semaglutide, it may not be medically suitable for them.“
LegitScript Flags Violative Merchants and Certifies Compliant Ones
LegitScript has flagged several merchant accounts found to be selling unapproved drugs containing semaglutide, often marketed as research chemicals. Among the most concerning were merchants selling unmarked bottles of white powder. Similar to weight-gain products such as Apetamin, these products are likely being marketed via social media accounts.
Conversely, LegitScript Certification helps healthcare and telehealth merchants demonstrate compliance. Our program provides a clear, verifiable signal that merchants operating in highly regulated industries like healthcare are adhering to the strict, often evolving regulatory guidelines and are complying with jurisdictional requirements. Contact us to learn more.
This post was updated in October 2024 to reflect the latest regulatory news.